Isabelle François, Ph.D.1,2, Nikos Xynos, Ph.D.1,3
1 Thar Process, USA
2 Chromisa Scientific, Belgium
3 Nomad Labs, Greece
Introduction
Solvents are used in large amounts in the chemical, pharmaceutical, food, and natural product industries. [1,2] In the search for environmentally friendly solvents, increasing attention is being paid to supercritical fluids (SFs) for a wide variety of applications. For instance, supercritical solvents are used in extractions [3-5], material processing [6], micronization (reducing particle size) [7,8], chemical reactions [9], cleaning [10], drying [11], and chromatography [12], among other applications. Supercritical fluids add a new dimension to conventional (liquid) solvents: the density-dependent solvent power. The density of SFs can be easily fine-tuned to process needs, with changes in temperature, pressure, and/or composition. Other important advantages of SFs are their very low surface tensions, low viscosities, and moderately high diffusion coefficients. The most widely applied solvent for both supercritical fluid extraction as well as chromatography (SFE and SFC, respectively) is supercritical carbon dioxide (CO2).
The emerging medicinal and recreational cannabis industry has ignited interest in using supercritical CO2 for the extraction and purification of cannabis/hemp flower and/or trim.
As more companies in this industry are transforming themselves with a high focus on sustainability and quality, the use of supercritical CO2 fits nicely into their business strategies.
CO2 reaches its supercritical state at the critical conditions (31.1°C and 73.8 bar). Figure 1 shows the CO2 phase diagram.
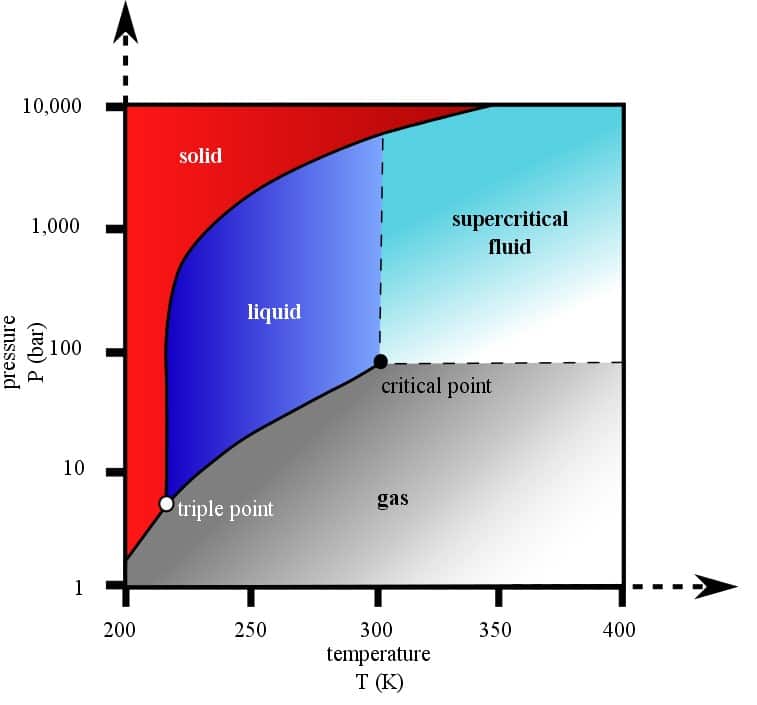
[Figure 1] CO2 phase diagram (Ben FinneyMark Jacobs, CC0, via Wikimedia Commons)
Advantages and limitations of SFC in natural product/cannabinoid purification
Supercritical CO2 represents significant advantages when used as an extraction and chromatography solvent, and this applies well for cannabinoids among other target compounds.
CO2 is non-flammable and physiologically compatible. It is also considered a green and sustainable solvent as it is environmentally neutral (recovered from industrial and fermentation plants). It, therefore, avoids producing CO2 that would have been generated from disposal of the solvents it replaces. Additionally, since CO2 expands to a gas when exposed to atmospheric pressure and at room temperature, extractions and purifications performed using supercritical CO2 leave little-to-no solvent in the extract and/or fractions, and therefore, typical evaporation times are significantly shortened or not necessary at all.
What’s more, CO2 can be recycled throughout the process, further minimizing solvent consumption. This means that the entire process is vastly cheaper and less laborious compared to liquid alternatives as the solvent, energy, and waste disposal-related costs are significantly minimized. This will quickly compensate the higher investment cost for the acquisition of the equipment.
One limitation regarding the use of supercritical CO2 as an extraction and/or chromatography solvent is that the related applications are limited as far as the extraction/isolation of very polar solutes is concerned due to the low polarity of the solvent. The possibility to utilize a co-solvent as modifier of polarity and solvent strength, or the usage of enhanced-fluidity liquid chromatography (EFLC) [13] that involves the addition of liquid CO2 to conventional liquid mobile phases, both compensate this limitation and empower the feasibility of a broad range of applications. In any case, the apolarity of the highly interesting and bioactive natural constituents of hemp and cannabis (e.g., cannabinoids and terpenes), means that SFE and SFC are well-fit technologies for these applications.
To demonstrate preparative (prep) and industrial SFC virtues by comparing various chromatographic solutions for their cost efficiency regarding the popular application of delta-9-tetrahydrocannabinol (THC) remediation from a broad-spectrum hemp extract, one needs to consider parameters such as:
1) CAPEX (capital expenditures) for the acquisition of the chromatography system as well as for the solvent recovery systems that will be dimensioned respectively to solvent consumption,
2) OPEX (operational expenses) for energy and staffing regarding chromatography and solvent removal operations,
3) OPEX associated to the lifecycle and replacing of stationary phase wherever this is solid and not liquid,
4) OPEX regarding liquid solvent consumption considering recyclability,
5) Throughput injection capacity per cycle,
6) Method duration that considers flow rates, stacked injections possibilities, etc.
It is thus a rather copious and laborious task producing an as-much-as-possible objective and representative technology juxtaposition, requiring the use of reliable data and a series of viable hypotheses. To do so, a study was produced leading to a juxtaposition Total Cost of Ownership (TCO) chart for a chromatography platform that will remediate THC from a broad-spectrum hemp extract. This chart remains a generic approach and is depicted in Figure 2.
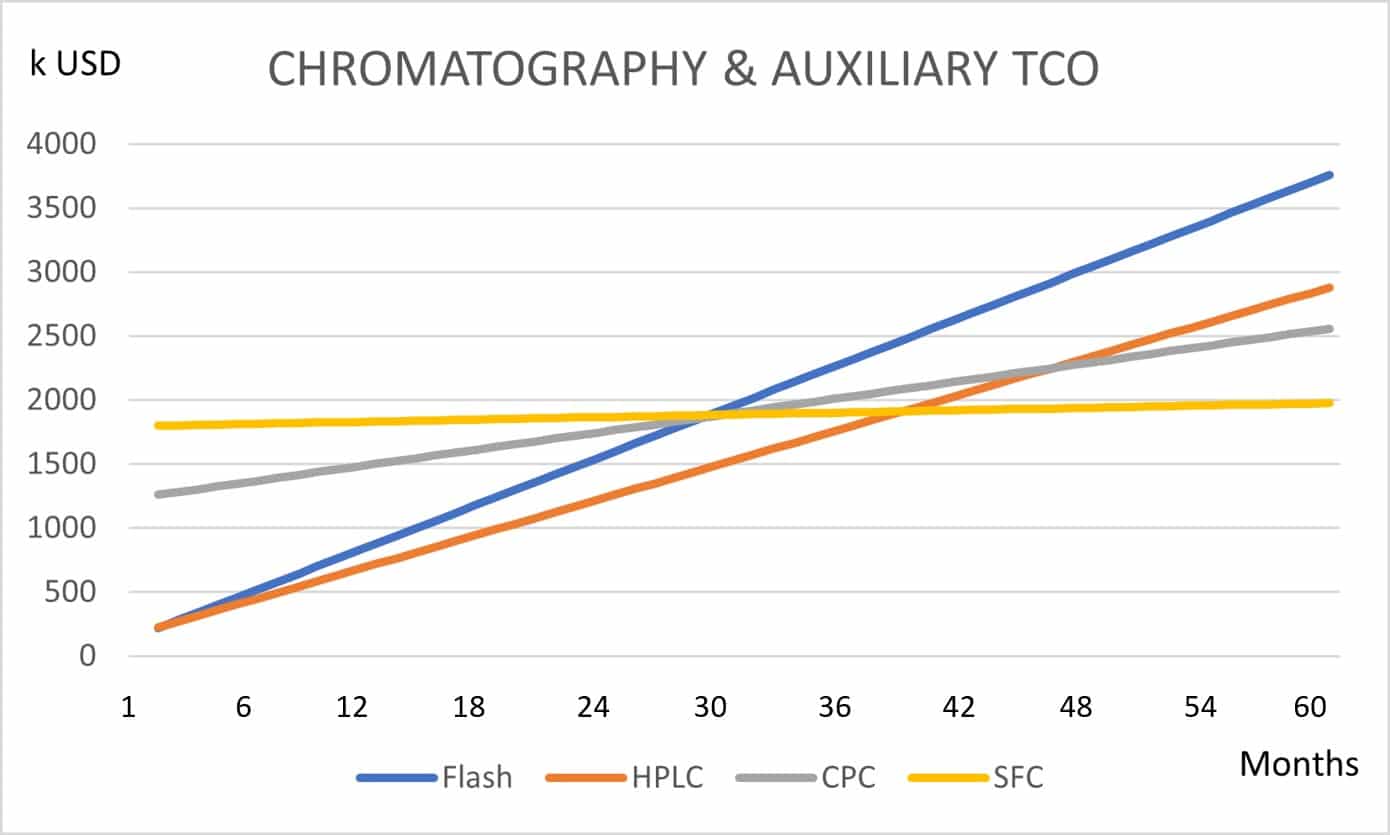
[Figure 2] Total Cost of Ownership juxtaposition of various chromatography platforms based on different technologies and THC remediation from a broad-spectrum hemp extract.
Industrial prep SFC for the cannabinoid industry is highly efficient for several strategies:
- Isolation of high-purity cannabinoids (cannabinoid isolates)
- Remediation of THC so that a broad-spectrum extract is obtained
- Pesticide remediation
- Acidic cannabinoid purification (directly after SFE/winterization)
SFC process flow and instrumentation
Prep SFC has been widely employed in many industries long before the cannabis business started booming. Prep SFC has been applied in natural product research and development, but predominantly within the pharmaceutical industry where the technique was and still is preferred over its liquid alternatives for the purification of small molecule pharmaceutical moieties, and even more importantly for the separation of enantiomers. [14-16]
In prep SFC, the sample (extract) is dissolved in ethanol (EtOH) and a large injection volume is injected into a chromatographic column which is packed with stationary phase. The mobile phase is supercritical CO2 mixed with small percentages of co-solvent (this application typically uses up to 2-3% EtOH when using the appropriate stationary phase). The mobile phase transports the different compounds that migrate across the column, and due to interactions of the compounds of interest between the stationary and mobile phase, separation occurs, and compounds elute at different retention times from the column. The detector keeps track of the elution pattern of the compounds and allows the collection system to collect fractions (isolated compounds) at specified timings.
When using supercritical CO2 as mobile phase, the system needs to be equipped with additional parts compared to a conventional liquid chromatography (prep LC) system. The flow diagram of a prep SFC system including recycling option is shown in Figure 3.
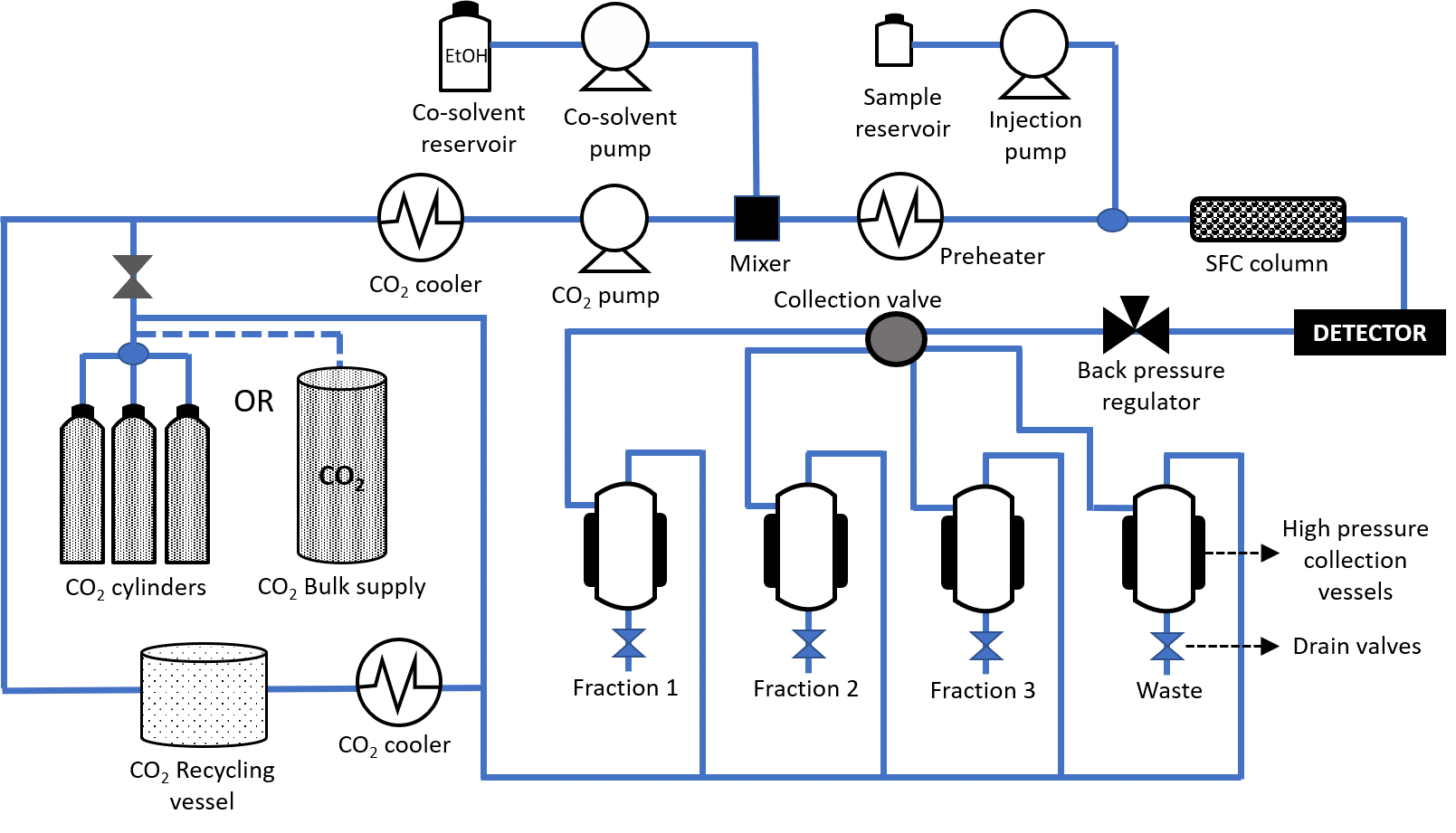
[Figure 3] Flow diagram of Prep SFC system including recycler.
In addition to a pump, injector, detector, recycler, and collection system, a prep SFC is also equipped with numerous heat exchangers to cool and heat the incoming CO2 at the right times in the process, as well as with a back pressure regulator (BPR). The heating device prior to the chromatographic column and the presence of the BPR enable the CO2 to turn into supercritical (or subcritical) state.
When applying isocratic conditions (i.e., maintaining a constant co-solvent percentage throughout the separation), stacked injections can be programmed which will further increase the throughput and productivity of the purification. The principle of stacked injections is shown in Figure 4.
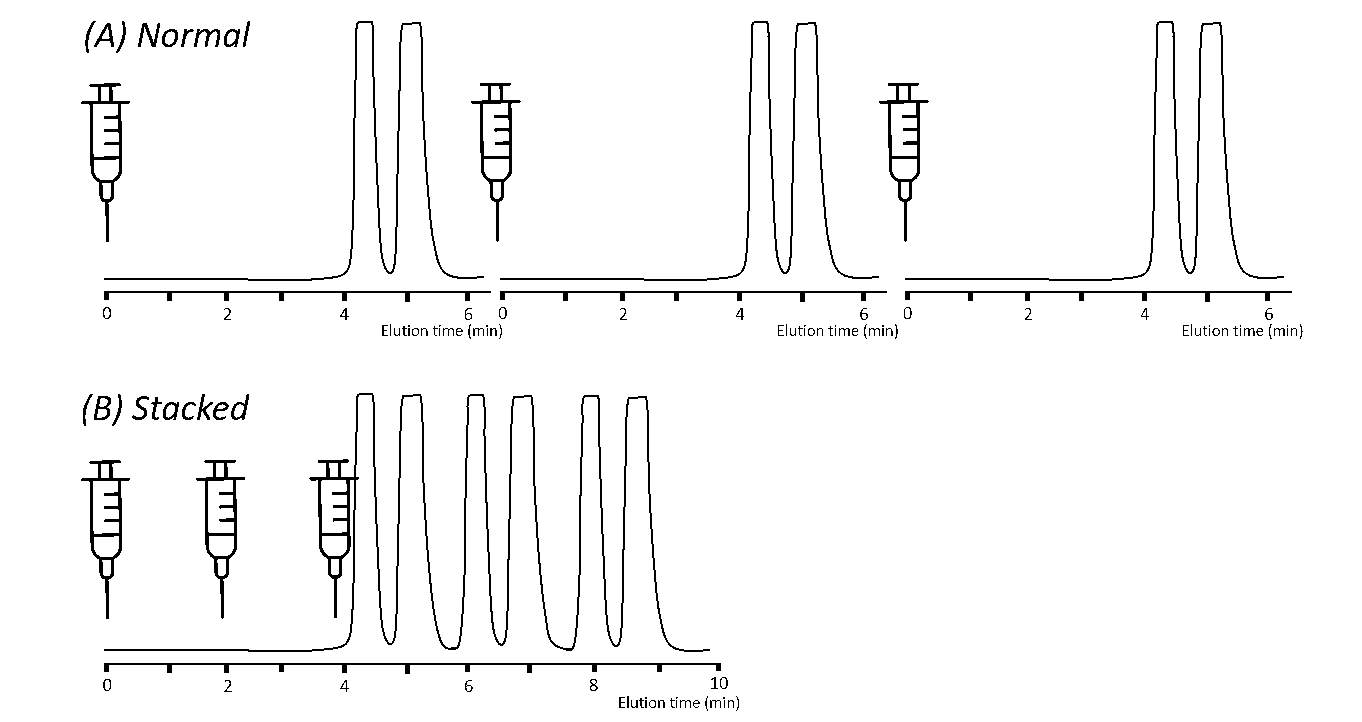
[Figure 4] Representation of the stacked injection principle
(A) Normal injection mode (B) Stacked injection mode
When performing purification by prep SFC, it is important to be cautious with the cannabinoid profile of the biomass starting material, as well as the ‘cleanliness’ of the injected sample. Lower complexity cannabinoid profiles addressing the target market from cultivation will enable higher productivities. Additionally, the presence of undesired species including waxes, chlorophyll, or other constituents that exist in the highly complex cannabis plant is to be avoided as they will impact the chromatographic profile and reduce throughput.
The followed extraction protocol and the processing after extraction will have a significant impact on the purification rate. SFE holds advantages in this respect due to the selective nature of the supercritical solvent, but even with this technology, minimum post-extraction processing is a must when purification is required. Typically, winterization followed by filtration and possibly color removal through activated carbon filtration will clean up the sample adequately, avoiding the copious and loss-associated steps of decarboxylation and short-path distillation.
Conclusions and Perspectives
Prep SFC, for the reasons stated above, represents a technologically and economically viable solution for both natural products and active pharmaceutical ingredient (API) purification. This makes it an appropriate solution for cannabinoid-related applications that usually fall between natural products and pharmaceuticals.
References
[1] Herrero M, Cifuentes A, Ibáñez E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae, and microalgae: A review. Food Chemistry. 2006;98:136-148. [journal impact factor 6.306; cited by 826][2] Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds. Editor: Martinez J. 2008. Boca Raton: CRC Press.
[3] King J. The relationship between cannabis/hemp use in foods and processing methodology. Current Opinion in Food Science. 2019;28:32-40. [journal impact factor 3.828; cited by 11]
[4] Mazzutti S, et al. Supercritical fluid extraction of Agaricus brasiliensis: Antioxidant and antimicrobial activities. The Journal of Supercritical Fluids. 2012;70:48-56. [journal impact factor = 3.744; times cited = 64]
[5] Díaz-Reinoso B, Moure A, Domínguez H, Parajó JC. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J Agric Food Chem. 2006;54(7):2441-2469. [journal impact factor 4.192; cited by 236]
[6] Cooper A. Recent developments in materials synthesis and processing using supercritical CO2. Advanced Materials. 2001;13:1111-1114. [journal impact factor 27.398; cited by 109]
[7] Kerc J, Srcic S, Knez Z, Sencar-Bozic P. Micronization of drugs using supercritical carbon dioxide. Int J Pharm. 1999;182(1):33-39. [journal impact factor 4.845; cited by 159]
[8] Reverchon E, Porta G. Supercritical fluids-assisted micronization techniques. Low-impact routes for particle production. Pure and Applied Chemistry. 2001;73:1293-1297. [journal impact factor 1.919; cited by 30]
[9] Licence P, Ke J, Sokolova M, Ross SK, & Poliakoff M. Chemical reactions in supercritical carbon dioxide: from laboratory to commercial. Green Chemistry. 2003;5:99-104. [journal impact factor 9.480; cited by 174]
[10] Weibel GL, & Ober C. An overview of supercritical CO2 applications in microelectronics processing. Microelectronic Engineering. 2003;65:145-152. [journal impact factor 2.305; cited by 143]
[11] Zhang JW, Liu HH, Yang H, Yang L. Drying characteristics of Eucalyptus urophylla × E. grandis with supercritical CO2. Materials (Basel). 2020;13(18):3989. [journal impact factor 3.057; cited by 3]
[12] Taylor L. Supercritical fluid chromatography. Analytical Chemistry. 2010;82(12):4925–4935. [journal impact factor 6.785; cited by 139]
[13] Bennett R, Biba M, Liu J, Ahmad IA, Hicks M, & Regalado E. Enhanced fluidity liquid chromatography: A guide to scaling up from analytical to preparative separations. Journal of chromatography A. 20191595:190-198. [journal impact factor 4.049; cited by 12]
[14] Phinney K. SFC of drug enantiomers. Analytical Chemistry. 2000:204A-211A. [journal impact factor 6.785; cited by 52]
[15] Miller L. Preparative enantioseparations using supercritical fluid chromatography. J Chromatogr A. 2012;1250:250-255. [journal impact factor 4.049; cited by 80]
[16] Guiochon G, Tarafder A. Fundamental challenges and opportunities for preparative supercritical fluid chromatography. J Chromatogr A. 2011;1218(8):1037-1114. [journal impact factor 4.049; cited by 157]
About Dr. Nikos Xynos:
Dr. Nikos Xynos is a Pharmaceutical scientist with PhD in Phytochemistry, focusing on Green & state-of-the-art extraction and purification technologies. He also has an MSc in drug and cosmetic formulation. Dr. Xynos has more than 15 years of experience in the field of botanical extraction, refining and purification for the pharmaceutical, nutraceutical, phytotherapeutic and cosmetics sectors. His technological expertise in terms of innovative processing technologies focuses on Supercritical CO2 Extraction (SFE), Subcritical Water Extraction (SWE), Microwave-assisted Extraction (MAE), Pressurized Liquid Extraction (PLE), Centrifugal Partition Chromatography (CPC), Supercritical Fluid Chromatography (SFC), Adsorption Resin Technology (ART), Molecular Distillation (SPD), Liquid/Liquid Extraction (LLE) and more. His most important experience lies in the project management of R&D from biomass sourcing to process development and technology transfer. Dr. Xynos holds several peer-reviewed scientific publications and is a reviewer for scientific journals as well as a speaker in scientific conferences & seminars/webinars.
Dr. Xynos acts worldwide as a Science & Technology advisor for the industry that focuses on the development of biologically active products based on botanicals & natural substances. He has implemented several R&D projects for the Natural Products & Cannabis Industries for key players in the market. His contribution as an advisor includes Process design, Conceptualization of processing units, Optimal technology sourcing, Development of international cooperation and partnerships at Science & Technology and Business level, Know-how transfer in terms of Intellectual/Industrial Property (IP), development of Standard Operating Procedures (SOP), Continuous process improvement and more.
About Isabelle François:
Isabelle François is the founder and director of Chromisa Scientific, a consultancy company based in Belgium. She supports academia as well as industry with demanding chromatography, purification and project management challenges, offering customization, training and support. Since October 2020, Isabelle works for Thar Process as business development manager for Europe, Middle East and Africa.
Isabelle conducted her PhD research in the lab of Prof. Pat Sandra at Ghent University in Belgium. During her research, she developed two-dimensional comprehensive fluid-based chromatography approaches (LCxLC and SFCxLC), focusing on the improvement of instrumental hardware. In 2009, she started working at ExxonMobil Chemical managing the global chromatography lab in the Advanced Characterization Department. Between 2011 and 2020, she worked for Waters in management and marketing roles, covering Europe and India. Her areas of expertise include UHPLC, SFC, preparative chromatography, SFE, comprehensive and heartcutting 2D chromatography (LC-LC, SFC-LC) with optical detectors and MS, as well as project management.
In 2014, she has been recognized by the magazine The Analytical Scientist as one of the most influential scientists under the age of 40 in their Powerlist, and she was part of the 2016 Powerlist as one of the most influential female scientists in the world. Isabelle is publishing and reviewing articles on a regular basis, and is often invited speaker at scientific conferences.




